Hey guys,
2023 is turning out to be a tough year to say the least.
Life sciences venture capital investment, initial public offerings (IPOs) and employment growth have all declined in 2023, although National Institutes of Health (NIH) funding is increasing (we'll see if the government stays open past the X date of June 6 to distribute it), and companies have large amounts of cash available for mergers and acquisitions. However, the FTC may have something to say if you are trying to up-size your merger. Sorry Amgen.
The good news is the top three life sciences markets of Boston/Cambridge, San Francisco and San Diego are still attracting funding, although less than before. The bad news is the rest of us may be waiting until the Fed rate goes down below 3%?
Speaking of fed rates...
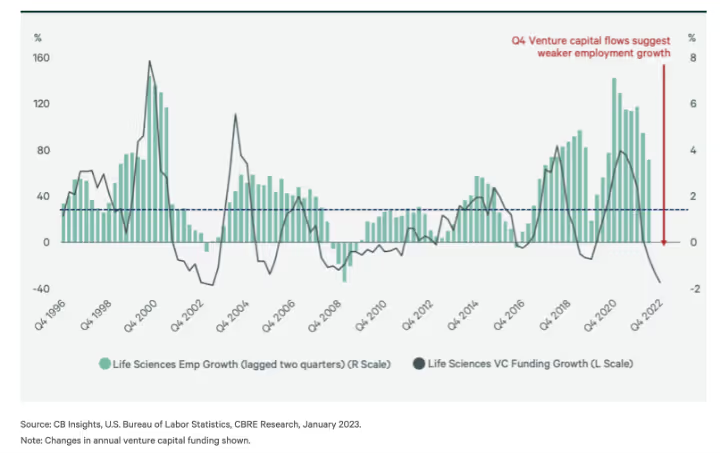
Note the negative growth in 2008.
At the end of 2007, the fed interest rate was at 4.5%.
Today it’s at 5.1%. Hmmm...
FDA "Encourages" De-Centralized Trials

The FDA has issued an update to a draft guidance on decentralized clinical trials (DCTs), which includes recommendations on design, the use of digital health technology, and recommendations on managing potential issues. Most folks in our industry hailed it as a big advance in adoption although I don't entirely agree. The guidance covers topics such as design considerations, remote visits, informed consent, safety monitoring, and more.
The draft explains that fully decentralized trials may be appropriate for investigational products that are simple to administer, have well-characterized safety profiles, and do not require complex medical assessments. However, certain parts of a trial may still need to be centralized. The FDA suggests discussing specific issues with relevant review divisions early on and emphasizes the importance of training, oversight, and risk assessment for successful implementation. The use of decentralized designs can increase diversity and improve recruitment, retention, and engagement of participants, particularly those in under-served communities.
The FDA will be accepting comments until August 1, 2023.
Need some FDA Guidance? Reach out to us here!
Money Walks
AstraZeneca is the third company to leave the Pharmaceutical Research and Manufacturers of America (PhRMA) lobbying group in the last six months, following AbbVie and Teva. They made the decision after assessing their membership and now desires to redirect their investment. Although it’s not clear many are citing PhRMA’s failed lobbying efforts to stop the passage of the Inflation Reduction Act, the Biden administration’s drug-pricing law that gives Medicare the power to negotiate certain drug prices. PhRMA has been pushing reforms to protect innovation and make health care more accessible and affordable, and recently launched an attack ad on pharmacy benefit managers.
The pharma lobby is strong with this one.
Clinical Trials... Harder Than It Looks
CVS Health has announced they will be shutting down their clinical trials business by the end of 2024. They will be trying to place affected employees in other parts of the company and have been streamlining their business with divestitures and other moves. The move is part of an effort to focus on their core business.
Their competitor, Walgreens, launched its own clinical trials division in June of last year. Retail giant Walmart and the grocery chain Kroger have also recently entered the space.
Disrupting the large players hasn’t been easy. Earlier this year, another new entrant, venture-backed Reify’s Care Access unit, announced major layoffs following problems with a Pfizer Lyme disease vaccine trial. This evolving landscape in the healthcare industry highlights the dynamic nature of clinical trials and the ongoing competition among key players.

Clinical Trial Help? Reach out to us!
My attempt at trying to be the marketing and advertising firm for Casana. Here goes nothing...
Looking to upgrade your bathroom experience?
Casana has got you covered with their latest invention - the Heart Seat! This smart toilet seat not only measures your oxygen saturation and heart rate, but it also plans to track your blood pressure in the near future.
No more worrying about taking your vital signs manually, the Heart Seat will do it for you in the comfort of your own bathroom. And the best part? It's a passive process, so you can continue your regular bathroom activities without having to change your behavior.
The Heart Seat analyzes your data and sends it to your care team's dashboard, designed in collaboration with Casana's partners. Now you can finally have reliable and clear health trends within a real-life context, and your care team can quickly react to any issues to keep you healthier at home. No word on if there is a warning indicator for one of those more challenging movements.
Casana plans to launch the Heart Seat by the end of 2023, so get ready to take a seat on the throne and let your toilet take care of your health.
Alternative working title was Medical Device Design in the Crapper.

Is Houston poised to unleash full potential as a prominent life sciences hub?
In this article from InnovationMap, Proxima's own Isabella Schmitt, MBA, RAC delves into Houston's promising prospects as a major player in the field of life sciences.
From funding challenges to talent acquisition and collaboration, Isabella highlights key concerns that require attention for Houston to truly shine in the world of MedTech and life sciences. It is a read that is absolutely worth your time.
The American Society of Clinical Oncology (ASCO) Annual Meeting is 6/2 - 6/6 in Chicago, will you be there?
-May-25-2023-08-07-17-7369-PM.avif)
This meeting is always very special and a fantastic opportunity to connect on the future of cancer research, something I am very passionate about. I will be there along with my colleagues Laura Wilson, Robbin Frnka, and Matt Wagener. If you'd like to schedule a meeting with me or either of us, we'd love to meet you! See you all in Chicago!
We love our clients and would love to hear from you. Let us know what you need or what you want to hear in the newsletter!
Kevin@ProximaCRO.com
Very truly yours,
Kevin
Subscribe to The Proxima Post!






